Over 73,000 get COVID-19 vaccines since Beijing begins inoculation
A total of 73,537 people in 220 inoculation venues across Beijing have received the first dose of COVID-19 vaccine in two days since the city began administering the jab among specific groups of people with higher infection risks on Friday.
A total of 73,537 people in 220 inoculation venues across Beijing have received the first dose of COVID-19 vaccine in two days since the city began administering the jab among specific groups of people with higher infection risks on Friday.
No serious side effects have been observed among the vaccine receivers, Gao Xiaojun, a spokesperson for the municipal health commission told a press briefing Sunday.
The inoculation currently mainly covers nine groups of people aged 18 to 59 in Beijing. These include frontline customs inspectors of imported cold-chain goods and personnel working in the overseas and domestic transportation sector.
A female staffer at a vaccination point in Beijing's Xicheng District was found waiting for recipients at 7 a.m. Sunday.
"I'm responsible for ID verification and registration before they get the vaccine," said the woman with face and hands turned red due to the cold. "Here we mainly serve practitioners in cold-chain and catering industries. Everyone queued up in an orderly way and fully understood and supported our work."
"Our company informed us of nine possible contraindications to receive the vaccine. Since I have none of them I came without hesitation," said Xie Fei, who works at a Walmart store in the district.
"The injection was administered in minutes and I have been observed for half an hour. Currently I don't have any discomfort," said Xie, who came with about 30 colleagues. "I'm frequently exposed to imported cold-chain food at work, and the COVID-19 vaccination not only protects me but also customers."
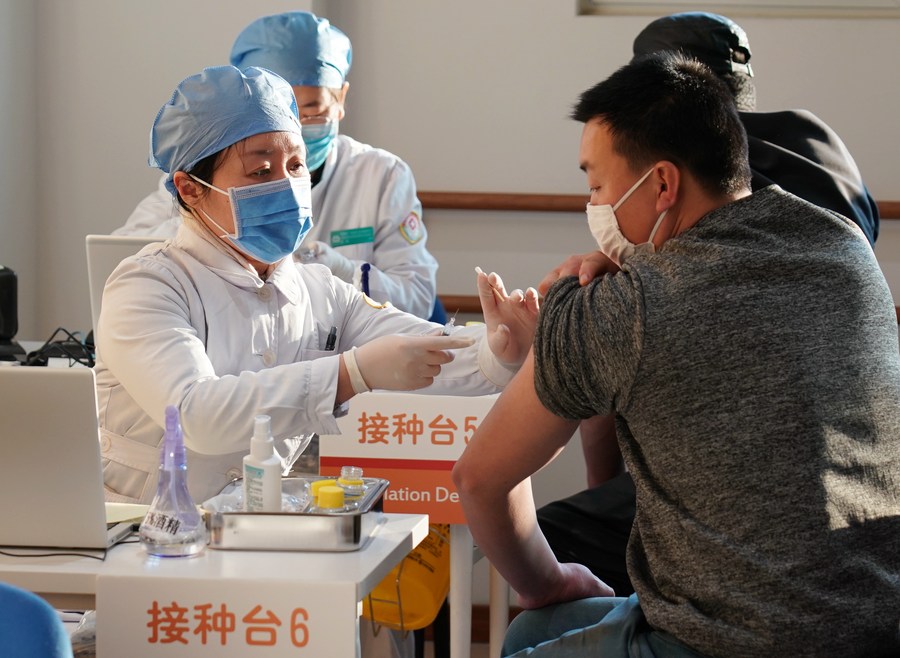
People are inoculated with the COVID-19 vaccines at a healthcare center in Honglian Community in Xicheng District of Beijing, capital of China, Jan. 3, 2021. Beijing has started administering COVID-19 vaccines among specific groups of people with higher infection risks. (Xinhua/Zhang Chenlin)
People receiving the jab will have the information updated on Beijing's health code app, Gao noted, adding that those who have urgent plans to travel overseas can apply for COVID-19 inoculation at the community service center.
After the vaccines receive market approval, authorities in Beijing will start vaccination for other groups on a reservation basis, said Gao at an earlier press conference.
China announced Thursday that it had granted conditional marketing authorization to its first self-developed COVID-19 vaccine. The inactivated vaccine got approval from the National Medical Products Administration. It was developed by the Beijing Biological Products Institute Co. Ltd. under the China National Biotec Group, affiliated with Sinopharm.

